Our group studies on organic synthetic chemistry. We are developing new synthetic methods for polyfunctionalized compounds. Group members are enjoying chemistry everyday.

If you want to know in detail, please go to SSP (Special Scholarship Program) page.
You can see Former and Current PhD Students of this group.
Projects
Our research is mainly focused on the chemistry of heterocyclic and nitro compounds. We are developing simple methods to construct skeletons that are not easily available by other methods, and synthesizing hitherto unknown frameworks. Through these investigations, we provide tools for researchers to use in applied research. Development of easy and efficient synthetic methods for a wide variety of skeletons will facilitate the search for new candidate compounds in the development of functional materials, such as pharmaceuticals, agrochemicals, dyes and optical materials.
Several research projects are shown below.
1) Development of highly efficient reaction through pseudo-intramolecular process(Details are Here)
2) Facile synthesis of polysubstituted pyridines(Details are Here)
3) Development of synthetic methods using diverse reactivities of a nitro group(Details are Here)
4) Development of functionalized synthetic unit(Details are Here)
5) Development of synthetic methods using vicinal tricarbonyl compounds(Details are Here)
6) Synthesis of polyfunctnionalized heterocyclic compounds by nucleophilic ring transformation(Details are Here)
7) Activation of an aromatic ring using steric repulsion of substituents(Details are Here)
Development of highly efficient reaction through pseudo-intramolecular process
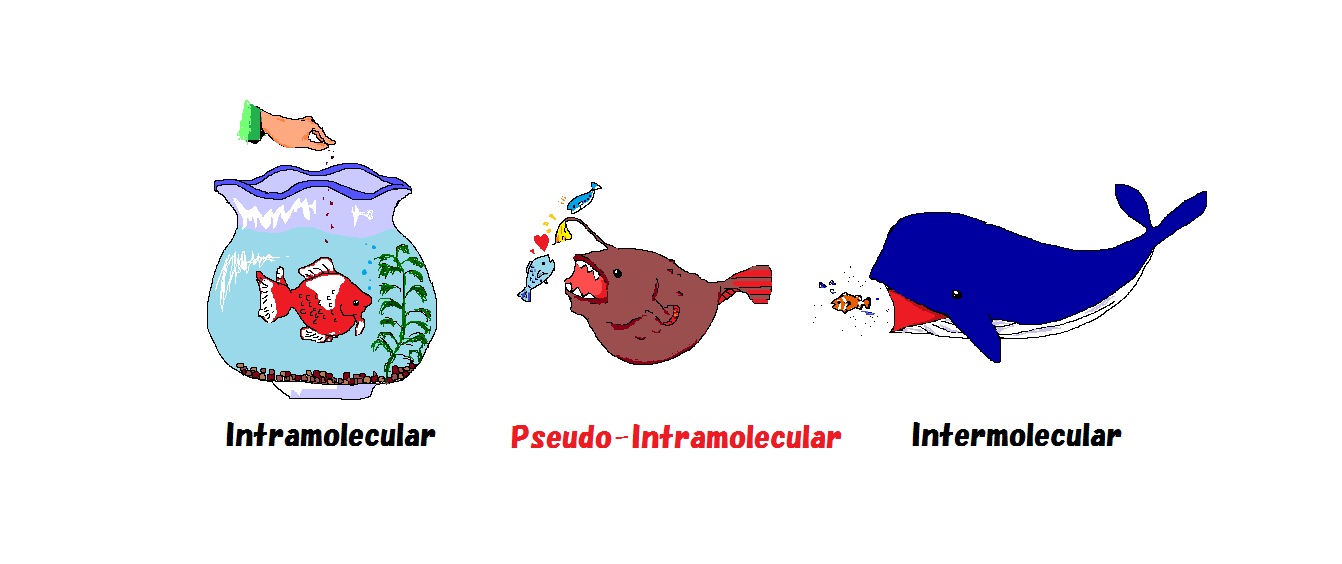
Developing highly efficient reactions is very important to reduce the environmental impact as it will save resources and energy. Eeactions usually proceed through intermolecular process, in which two reagents of moderate energy are required to meet and collide at a suitable angle. In contrast, intramolecular reactions proceed thousands of times faster than intermolecular reactions due to their high collision frequency. The intermolecular reaction is like a whale chasing small fishes in the ocean, and the intramolecular reaction is like a goldfish getting foods easily in an aquarium. On the other hand, the pseudo intramolecular reaction developed in our laboratory attracts a substrate to be reacted with highly acidic hydrogen to undergo the reaction, hence the reaction proceeds efficiently like an intramolecular reaction, even though it is an intermolecular reaction, it is like an angular fish.
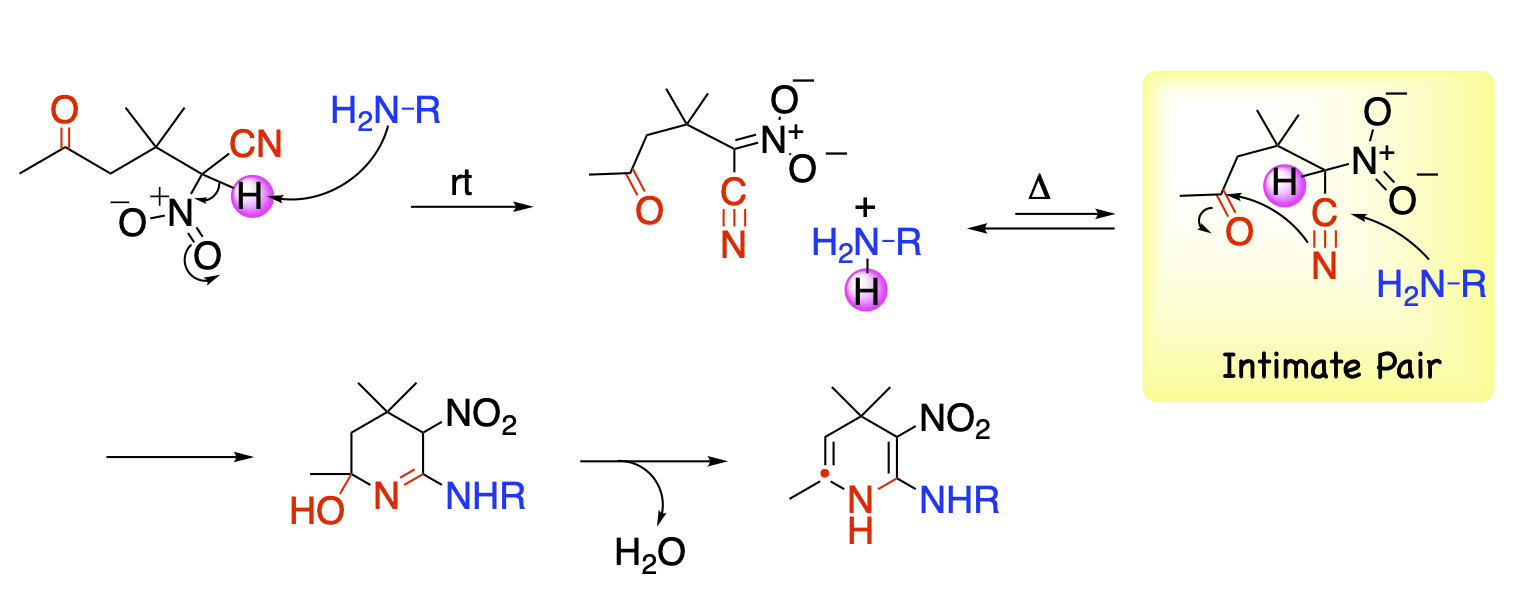
Compounds with both a highly acidic hydrogen and a functional group serve as substrates for this reaction. α-Nitro-δ-keto nitrile is a compound satisfying these criteria, which forms a salt immediately upon treatment with an amine. When this salt is heated, the amine is liberated under equilibrium. The close proximity of the nucleophile amine and the electrophilic keto nitrile facilitates the attack to the nearby cyano and then carbonyl groups, giving 1,4-dihydropyridine possessing an amino and a nitro groups at the vicinal positions.
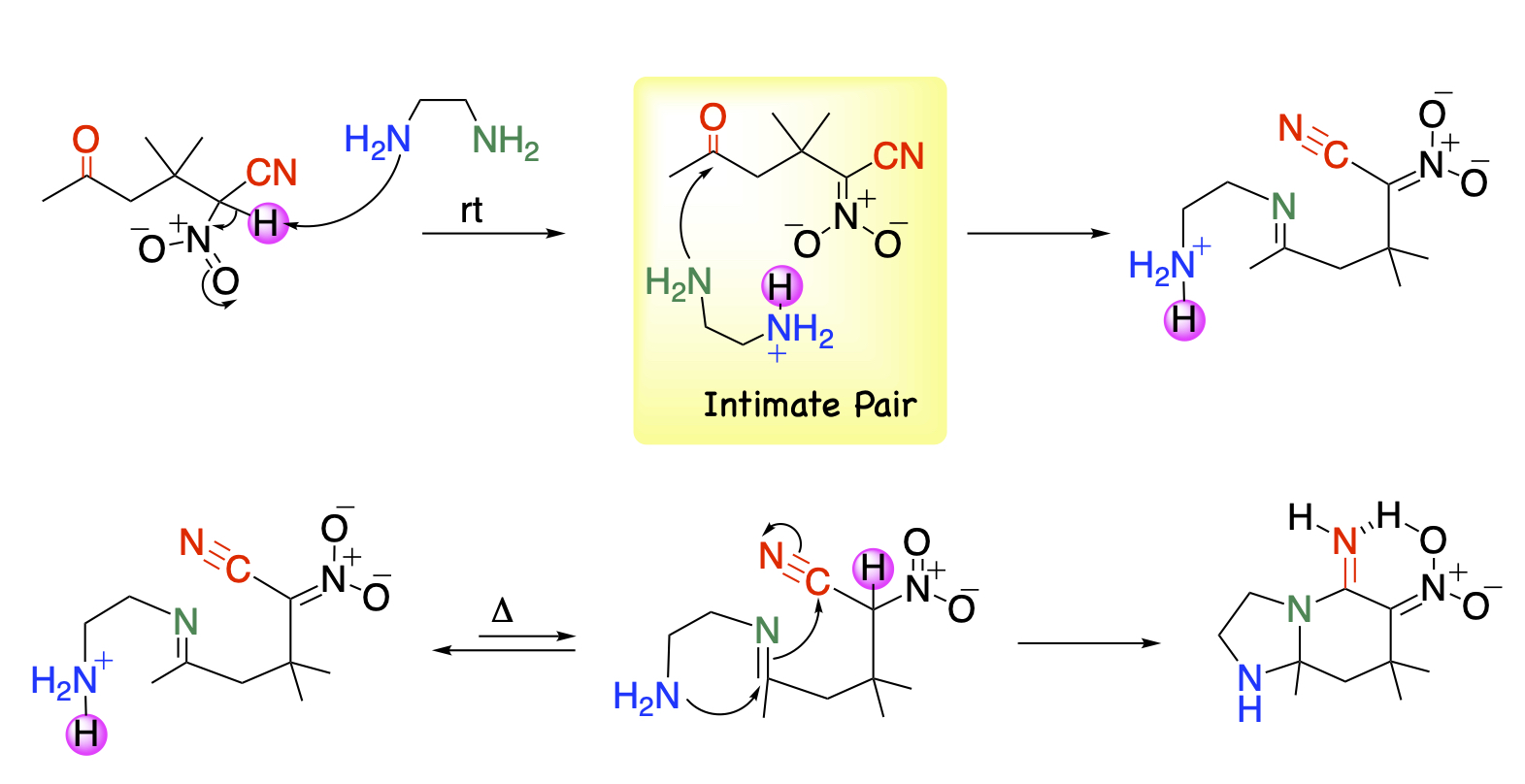
On the other hand, imine is efficiently formed upon treatment with diamine. When the other amino group is liberated, it attacks the imino group and then Oyano group to afford diazabicyclic compounds readily.
This method based on new concept not only construct polyfunctnionalized frameworks but also suppress side reactions because reactants are closed together.
Eur. J. Org. Chem. (2021)
Eur. J. Org. Chem. (2019)
Synthesis (2016)
Tetrahedron (2014)
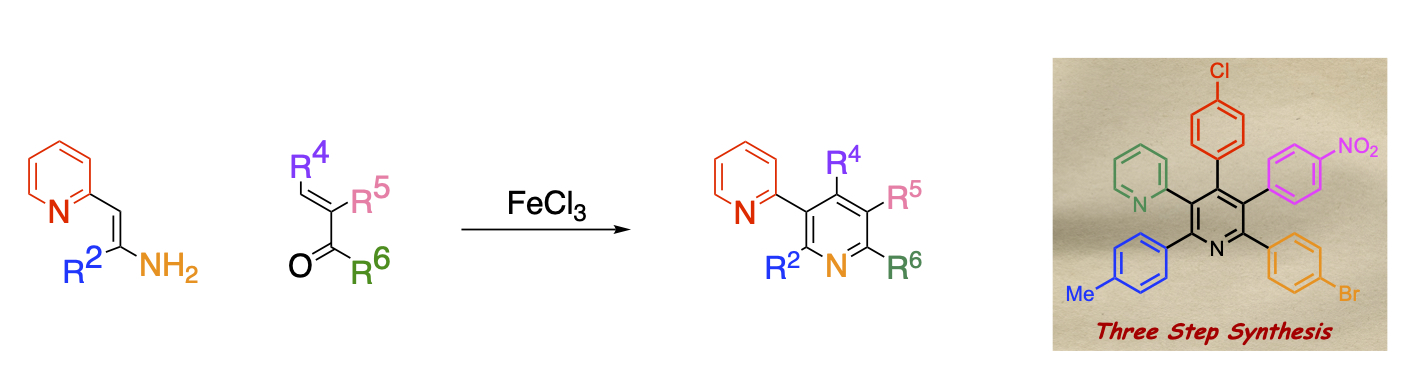
Facile synthesis of polysubstituted pyridines
Pyridine derivatives are often found as the basic skeleton of various functional materials. However, due to the lack of reactivity of the pyridine ring, it is difficult to introduce substituents, hence, even simple skeletons are often hitherto unknown. In particular, it is not easy to introduce alkyl and aryl groups. Therefore, the development of a simple method to introduce a desired substituent into a desired position is one of the most important issues.
We have found that the condensation reaction of enamino esters and α,β-unsaturated carbonyl compounds, which have both electron-donating and electron-withdrawing groups and biased electron density of the double bond. The reaction proceeds to give polysubstituted nicotinates upon heating in the presence of iron chloride. The advantage of this reaction is that the modification of the pyridine ring can be easily carried out simply by changing the enamino ester and the unsaturated carbonyl compound of the substrate. Furthermore, condensation between the ester functionality and the adjacent benzene ring can yield onicin and its derivatives, which show strong biological activity.
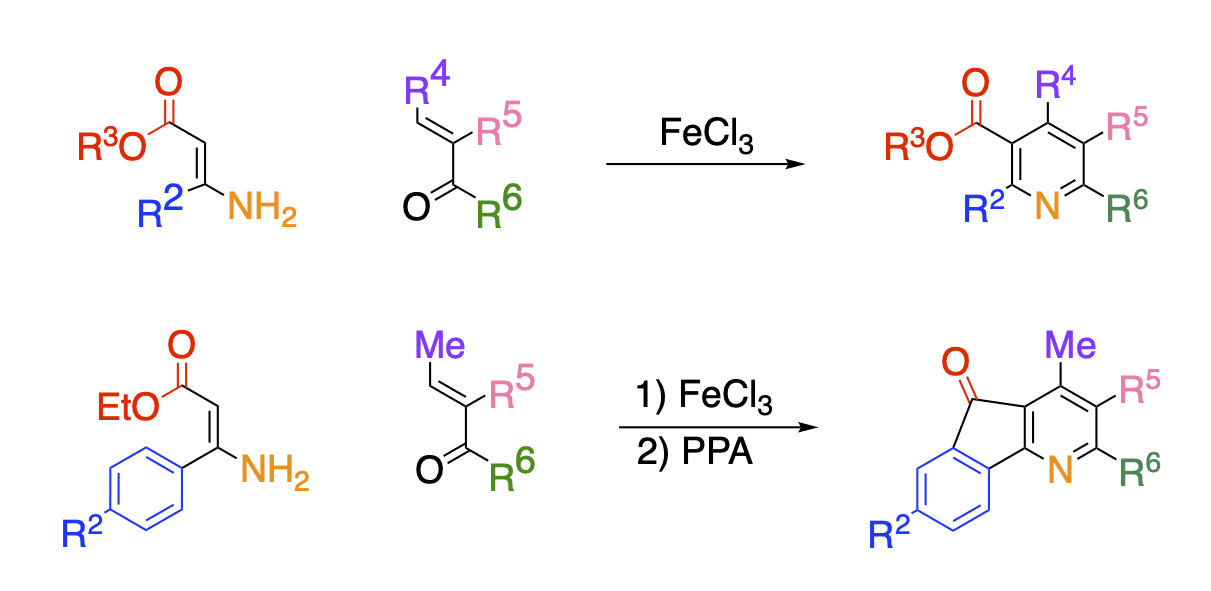
It is also possible to use enamines having a pyridyl group instead of ester groups. The synthesis of pyridines with different aryl groups at all positions requires multiple steps, but we have succeeded in synthesizing pyridines in three steps, including the synthesis of the raw materials, by using this method.
Recent Publications
Eur. J. Org. Chem. (2020)
Synthesis (2019)
Chem. Commun. (2017)
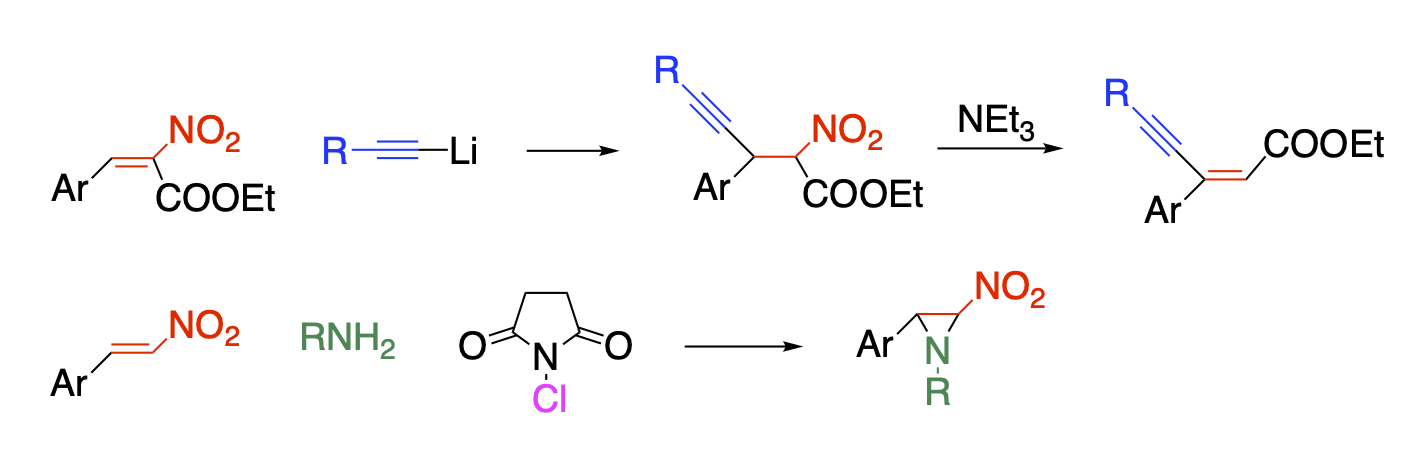
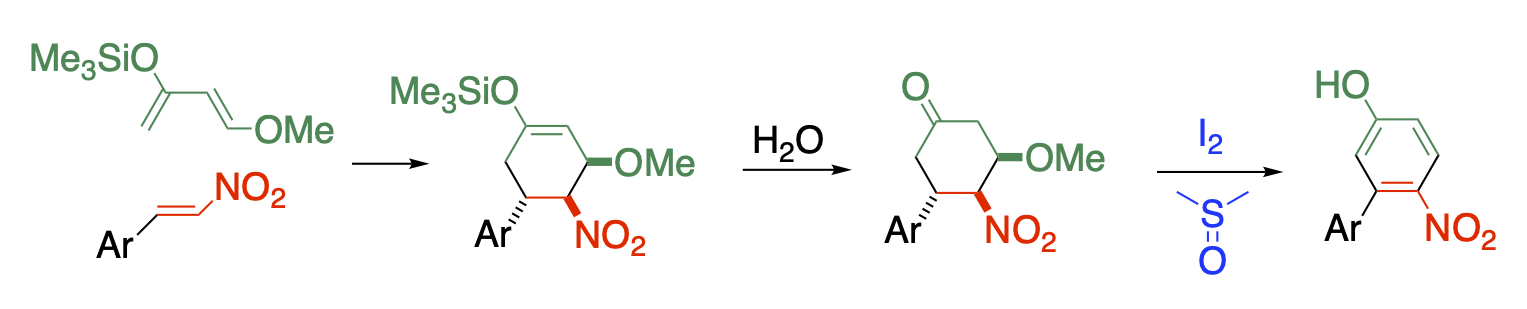
Development of synthetic methods using diverse reactivities of a nitro group
The nitro group shows strong electron-withdrawing properties, both in terms of induction and resonance, which reduces the electron density of the scaffold framework to increase its reactivity with nucleophilic reagents. Nitro group also increases the acidity of the α-hydrogen, and the deprotonated nitronate ion shows a duality of reacting with both nucleophilic and electrophilic reagents, which bring about behind the diverse reactivity of nitro compounds. The nitro group serves as a good leaving group to be substituted directly or to be eliminated as a nitrous acid with the adjacent hydrogen forming a double bond. Furthermore, chemical transformation of nitro groups can lead to compounds with other functional groups, and some nitro-containing compounds themselves show biologically activity. Combining these diverse reactivity makes it possible to synthesize multiply functionalized compounds.
Nitroalkene is a suitable substrate for such purpose. It is possible to functionalize the β-position when it is subjected to Michael addition followed by elimination of nitrous acid. Vicinal functionalization is also possible when the reaction is conducted in the presence of an electrophile. Polyfunctionalized benzene can be synthesized by Diels-Alder reaction.
Recent Publications
Beilstein J. Org. Chem. (2020)
Molecules (2020)
J. Org. Chem. (2018)
Org. Lett. (2017)
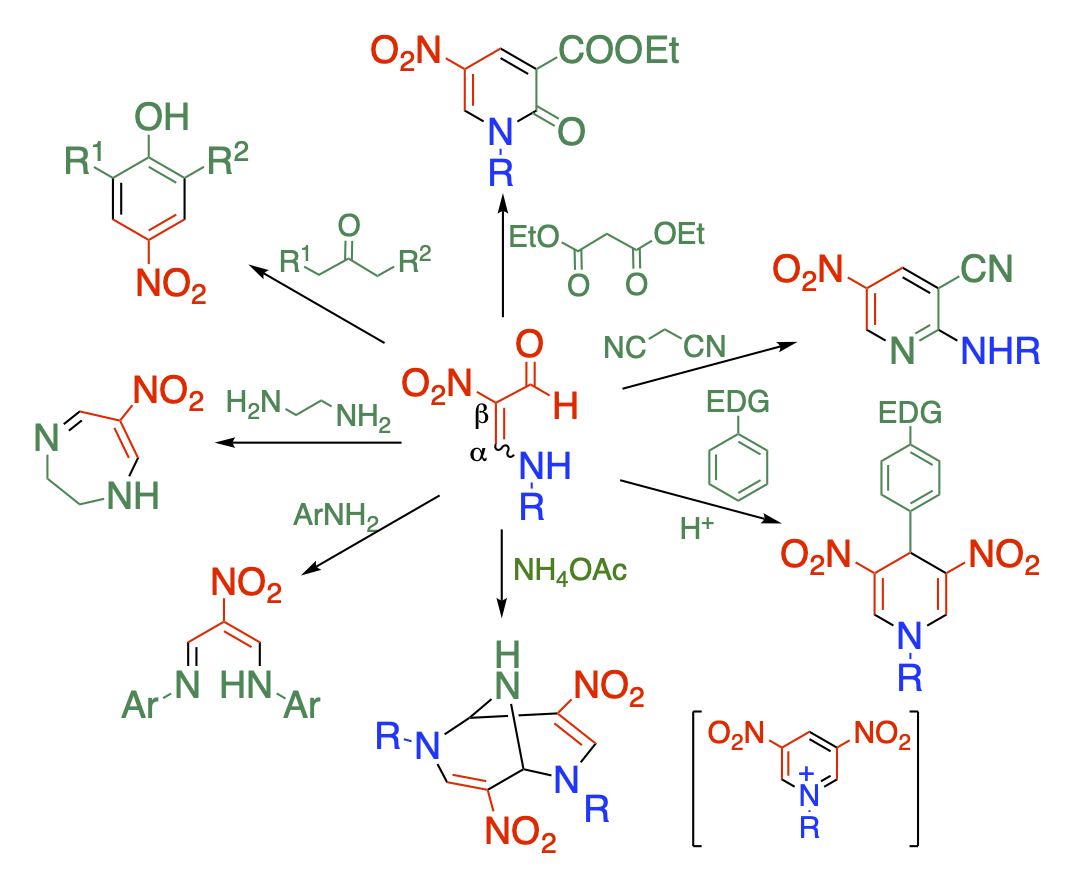
Development of functionalized synthetic unit
There are several methods to synthesize multiply functionalized compounds. Built-in method of a functionalized synthetic unit is one of these methods. We have developed several units and have used them in organic synthesis.
Formylnitroenamines, which contain nitro, formyl, and amino groups, are multi-functionalities and show versatile reactivities. This structural feature enables the synthesis of a wide variety of frameworks. By using a reagent with two nucleophilic sites, a five-membered ring pyrazole, a six-membered pyrimidine, and a seven-membered diazepine can be obtained. When a reagent possessing both a nucleophilic and an electrophilic moiety is applied, polyfunctnionalized pyridones and aminopyridines can be formed. The self-condensation affords dinitropyridinium ions and bicyclic skeletons under acidic conditions.
Recent Publications
Org. Biomol. Chem. (2020)
Russ. Chem. Bull. Int. Ed. (2016)
RSC Adv. (2015)
Although the pyridinium ion of nitroisoxazolone is an anionic compound, it readily undergoes the ring-opening reaction upon treatment with a base to give cyano-aci-nitroacetate. The dianionic property and multi-functionality facilitate to react with carbonyl and unsaturated carbonyl compounds to give isoxazole and naphthyridine derivatives. In this case, cyanoacinitroacetate can be used as synthetic equivalent of explosive nitroacetonitrile. We have also found that it serves as a 1,3-dipole under acidic conditions to undergo cycloaddition reactions.
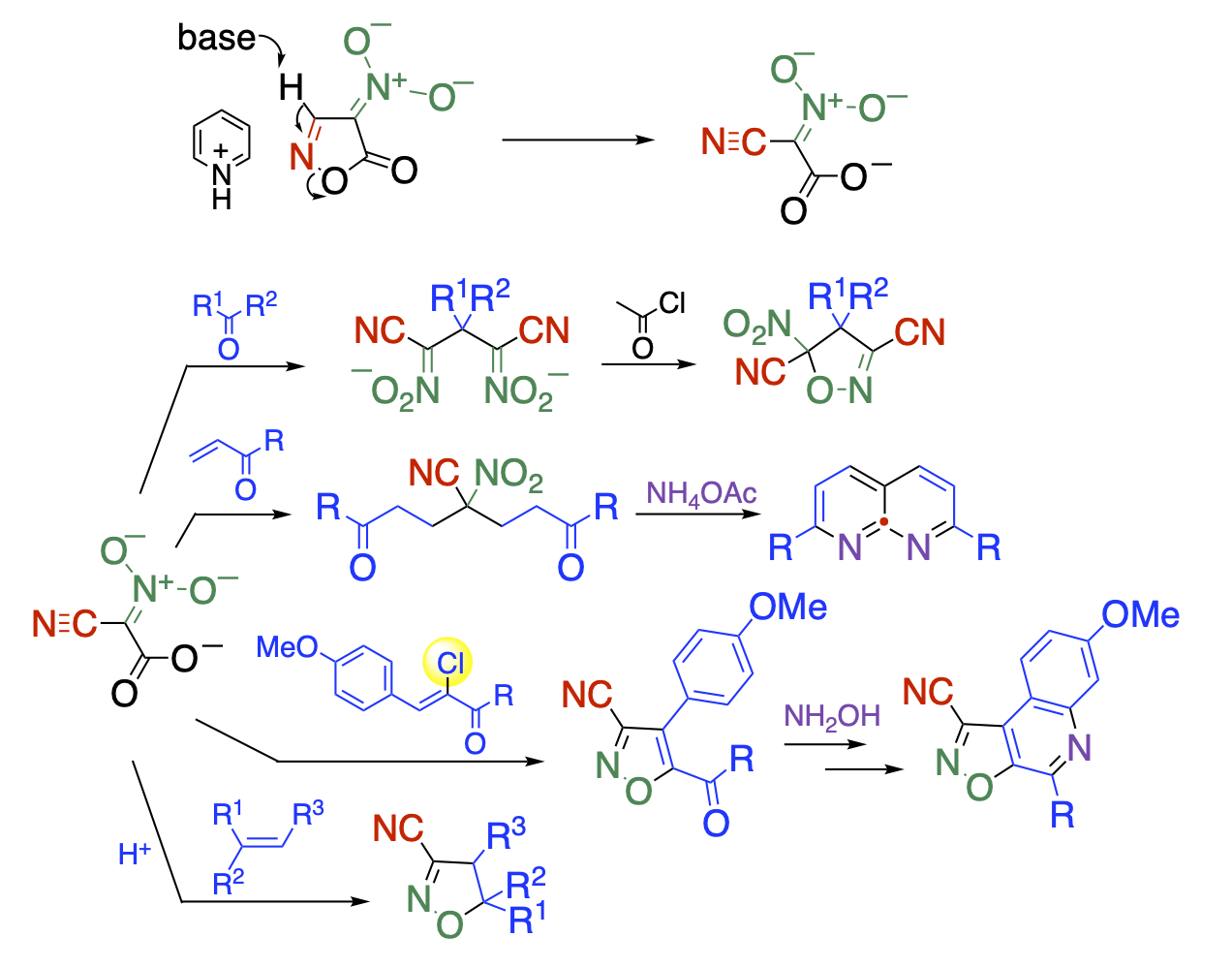
Recent Publications
J. Org. Chem. (2021)
Chem. Commun. (2019)
Curr. Med. Chem. (2017)
J. Org. Chem. (2017)
On the other hand, N-methylnitroisoxazolone readily undergoes the ring opening reaction upon treatment with water to generate nitrile oxide possessing a carbamoyl group. Not only alkenes and alkynes, but also electron-rich nitriles react with the nitrile oxide to afford the corresponding cycloadducts. The active methylene compounds also serve as dipolarophiles, which proceeds the cycloaddition with the nitrile oxide successfully when chelate complexes with magnesium is formed to fix them in the enol form. The functional group of the cycloadducts is limited to the N-methylcarbamoyl group. This problem is overcome by tosylation, which serves like a Weinreb amide and can be converted to various functional groups. As a result, the carbamoylnitrile oxide is regarded as a synthetic equivalent of a variety of functionalized nitrile oxides.
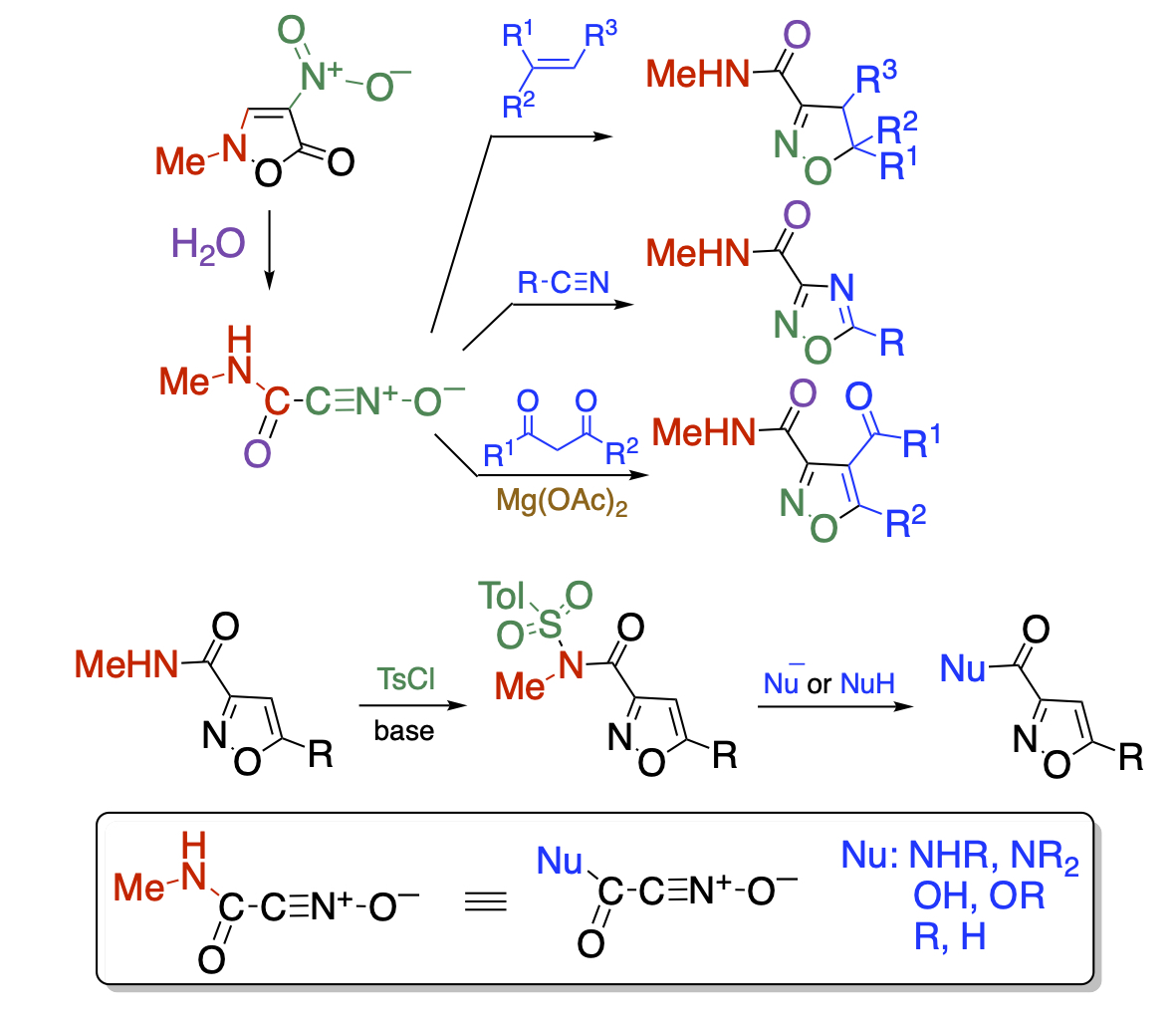
Recent Publications
Beilstein J. Org. Chem. (2015)
Methods and Applications of Cycloaddition Reactions in Organic Syntheses (2014)
Org. Bioorg. Chem. (2012)
Development of synthetic methods using vicinal tricarbonyl compounds
Compounds with three consecutive carbonyl groups have high reactivity and are useful as synthetic blocks for obtaining multiply functionalized compounds. We have studied diethyl mesoxalate (DEMO), which is commercialized by Kumiai Chemical Industry. The central carbonyl group is highly electrophilic to react with less nucleophilic reagents such as acid amides to give N,O-hemiacetals. When the reaction is carried out in acetic anhydride, O-acetylation proceeds at the same time to afford N,O-acetal in a single step. The subsequent treatment with base causes elimination of an acetic acid leading to N-acylimine.
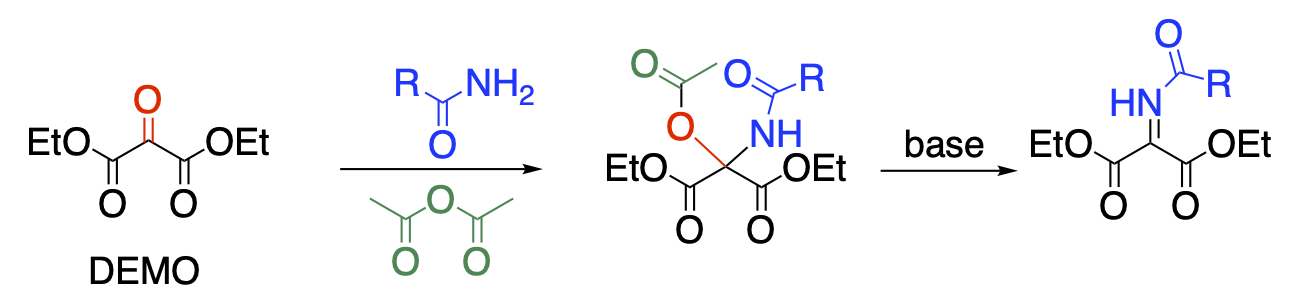
Recent Publications
Curr. Org. Chem. (2019)
Bull. Chem. Soc. Jpn. (2018)
Adv. Synth. Catal. (2016)
Synthesis of polyfunctnionalized heterocyclic compounds by nucleophilic ring transformation
While Diels-Alder and degenerate ring transformations have been actively studied for a long time, nucleophilic ring transformation has not been enough studied. We have focused on the structural features of dinitropyridone. It is not only highly electron-deficient due to the presence of a nitro group, ring nitrogen and a carbonyl group, but also has a good leaving group of nitroacetamide as a partial structure. This compound is a suitable substrate for nucleophilic ring transformation. When dinitropyridone is subjected to the reaction with a ketone as a nucleophile in the presence of ammonium acetate as a nitrogen source, three-component ring transformation proceeds to afford functionalized heterocyclic compounds that are not easily available by alternative methods. In these series of reactions, dinitropyridone serves as a safe synthetic equivalent of unstable nitromalondialdehyde.
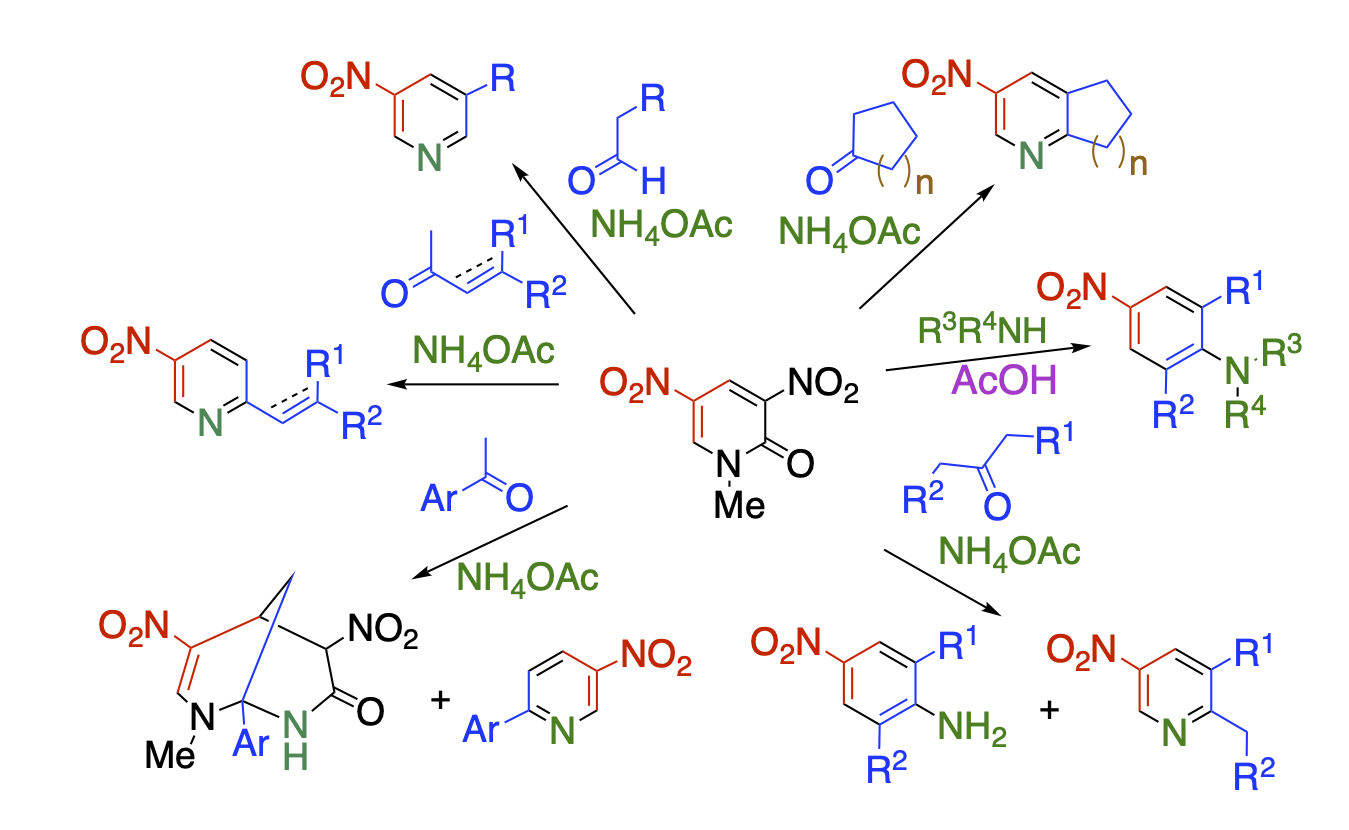
Recent Publications
有機合成化学協会誌 (2016)
J. Org. Chem. (2015)
Eur. J. Org. Chem. (2015)
Nitropyrimidinone, in which one of the nitro groups of dinitropyridone is replaced by a ring nitrogen, is also an excellent substrate for nucleophilic ring transformation. In this case, pyrimidinone serves as a synthetic equivalent of activated diformylamine and α-nitroformylacetic acid.
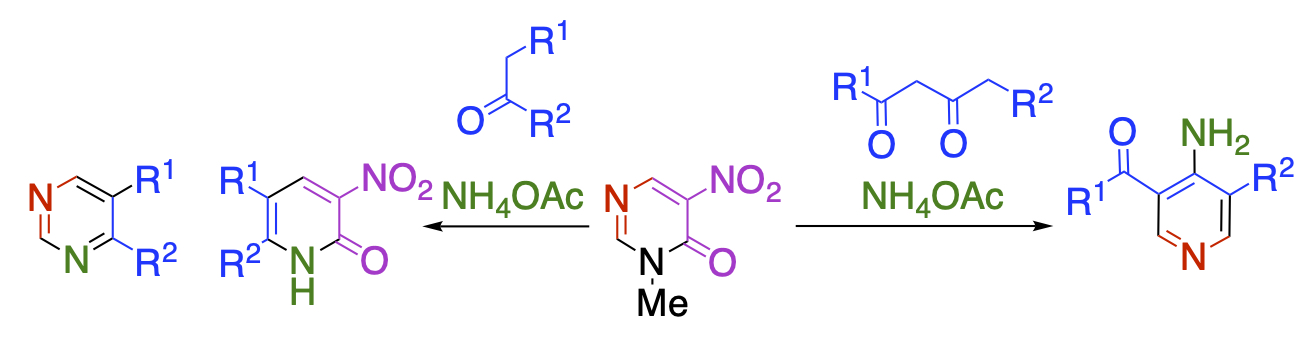
Recent Publications
Molecules (2021)
Tetrahedron Lett. (2013)
Activation of an aromatic ring using steric repulsion of substituents
Because aromatic rings are highly stabilized, the aromaticity should be disrupted for the modification. Usually, aromatic rings are activated by the electronic effects of substituents or by introducing a halogen of which carbon-halogen bond is used. Our research is based on the idea that a simple steric repulsion between substituents can destroy co-planarity and reduce aromatic stabilization. Indeed, the introduction of alkyl groups of various sizes into the 8-position of the 1-methylquinolinium salt for comparison revealed that co-planarity collapses and reactivity increases as the 8-substituent becomes bulkier.
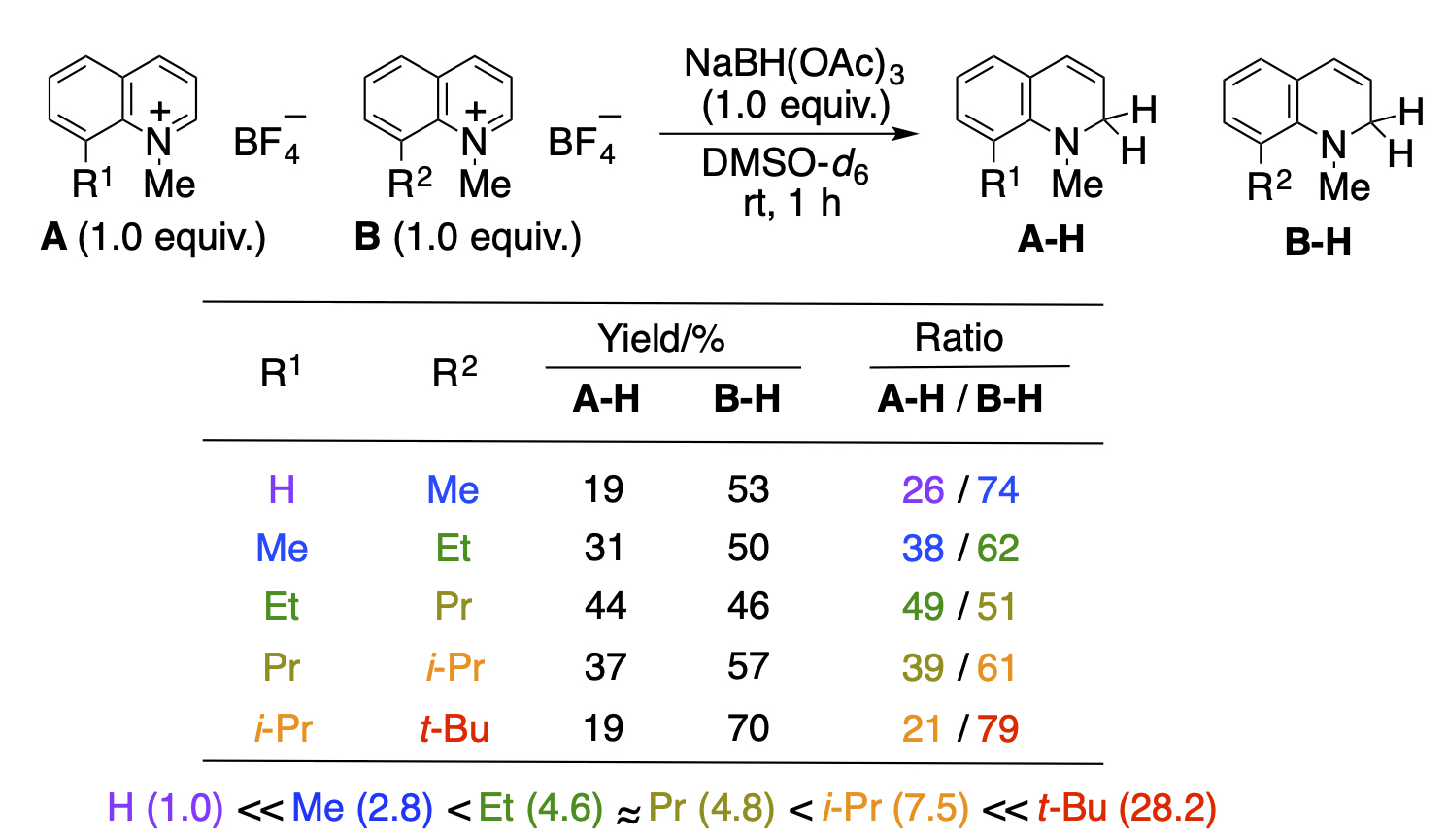
Recent Publications
Bull. Chem. Soc. Jpn. (2020)
Tetrahedron (2013)